0.9 Sodium Chloride Injection Usp 10ml
SODIUM CHLORIDE USP 09 FOR INJECTION 10ML LUER LOCK PLASTIC AMPOULE NO PRESERVATIVE. Care is also required with administering this solution to very young or to elderly patients.

Sodium Chloride Injection Usp 0 9 Single Dose Ampules
Electrolytes per 1000 mL.
0.9 sodium chloride injection usp 10ml
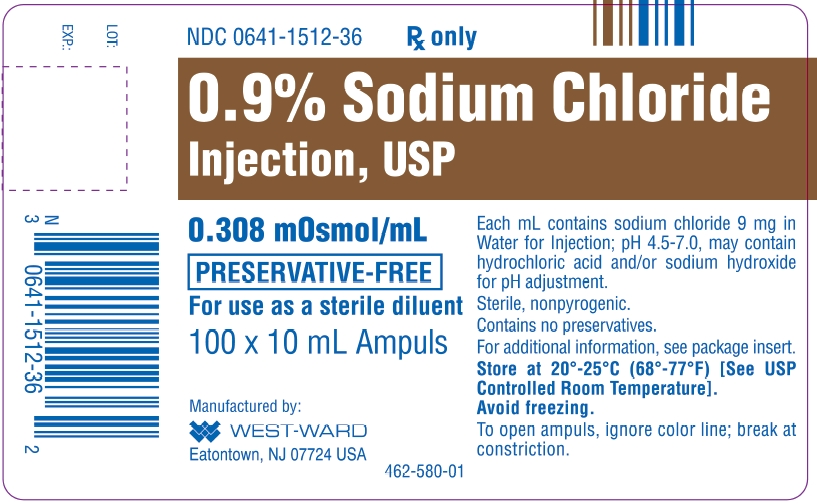
. SODIUM CHLORIDE INJECTION USP 09. Each mL contains sodium chloride 9 mg. Each mL contains sodium chloride 9 mg. Supplied only in single-dose containers to dilute or dissolve before injection.It contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection. 09 Sodium Chloride Injection USP is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. 09 Sodium Chloride Inj. Hospira Sodium Chloride for Injection is a sterile nonpyrogenic isotonic solution of sodium chloride and water used for injection.
Each mL contains sodium chloride 9 mg. Each mL contains sodium chloride 9 mg. It contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection. Sodium Chloride Injection USP 09 is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection.
USP 50 mL Sodium Chloride Injections USP are sterile nonpyrogenic isotonic and contain no bacteriostatic or antimicrobial agents. 09 Sodium Chloride Injection USP is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. 09 Sodium Chloride Injection USP contains no preservatives. Each mL contains sodium chloride 9 mg.
25 single-use vials per traySodium Chloride for Injection 09 Sterile No. 09 Sodium Chloride Injection USP is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. For 09 Sodium Chloride Injection USP each 100 mL contains 900 mg sodium chloride in water for injection. Each mL contains sodium chloride 9 mg.
Material Safety Data Sheet. Each mL contains sodium chloride 9 mg. Used to dilute and dissolve liquids and reduce the strength before you injection. 09 Sodium Chloride Inj.
Each mL contains sodium chloride 9 mg. 09 Sodium Chloride Injection USP solution is sterile and nonpyrogenic. Neat to watch it drip. Sodium Chloride Injection USP 09 is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection.
The pH is 53 45 to 70. Each mL contains sodium chloride 9 mg. VID 20170103 093327 0 9 Sodium Chloride Injection USP IV dripIntravenous drip at CHOP hospital. The osmolarity is 308 mOsmolL calc.
09 Sodium Chloride Injection USP is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. 09 Sodium Chloride Injection USP solutions are sterile and nonpyrogenic. Sodium Chloride NACL for Injection 09 is a sterile solution packaged in a flip top plastic vial. For 09 Sodium Chloride Injection USP each 100 mL contains 900 mg sodium chloride in water for injection.
09 Sodium Chloride Injection USP is indicated for extracellular fluid replacement treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration. Sodium Chloride Injection USP 09 5 mL and 10 mL Single-Dose Ampules. It contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single dose ampules.
For 09 Sodium Chloride Injection USP each 100 mL contains 900 mg sodium chloride in water for injection. It contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single-dose. 09 Sodium Chloride Injection USP solution is sterile and nonpyrogenic. Sodium Chloride Injection BP 09 wv should be administered with caution to patients with congestive cardiac failure pre-eclampsia impaired renal function or oedema with sodium retention.
09 Sodium Chloride Injection USP is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. It contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection. They are parenteral solutions containing various concentrations of sodium chloride in water for injection intended for intravenous administration. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration.
Not manufactured with latex PVC or DEHP. 09 Sodium Chloride Injection USP contains no. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes and water for hydration. 9 mgmL x 10 mL Polyampoule.

Sodium Chloride Injection Usp 0 9 10ml

Sodium Chloride Injection Usp 0 9 Single Dose Ampules

0 9 Sodium Chloride Injection Usp 10ml Single Dose Fliptop Vial 25 Vials Ddp Medical Supply

0 9 Sodium Chloride Injection Usp Med Plus Physician Supplies

0 9 Sodium Chloride Injection Usp
Bacteriostatic Sodium Chloride Usp 0 9 10ml 30ml Farris Laboratories Inc

Diluent Sodium Chloride Preservative Free 0 9 Intramuscular In

Sodium Chloride For Injection 10ml 25 Tray Mountainside Medical Equipment
Post a Comment for "0.9 Sodium Chloride Injection Usp 10ml"